Samsung Receives FDA Clearance for AI Algorithms that Detect Lung Nodules in Chest X-rays

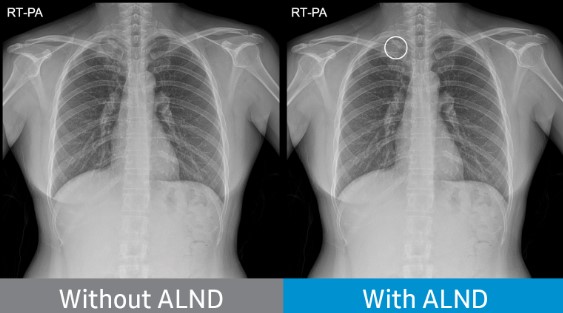
Chest radiograph without Auto Lung Nodule Detection (ALND) and chest radiograph with lung nodule marked.
DANVERS, Massachusetts – October 28, 2021 – NeuroLogica Corp. today announced it has received Food and Drug Administration (FDA) 510(k) clearance for its Auto Lung Nodule Detection (ALND) tool. The offering provides an on-device, computer-assisted detection (CADe) solution for detecting pulmonary nodules from 10 to 30mm in size through an artificial intelligence (AI) algorithm.*
It is designed to aid the physician in reviewing PA chest radiographs of adults and is part of S-Station, an operation software installed on Samsung Digital X-ray Imaging systems.
“This FDA clearance is a huge milestone for Samsung and is the result of our tireless work to design diagnostic solutions that empower providers to deliver patients the absolute best care possible,” said David Legg, Vice President of Digital Radiography and Ultrasound at Samsung NeuroLogica. “The fact that it delivers clinically reliable results means clinicians can present it to patients with the utmost confidence, and for that we’re very proud.”
Benefits of ALND include:
- Aiding the reader's diagnosis by indicating the location of suspected lung nodules on chest X-ray images (posteroanterior chest radiographs). The deep-learning technology has been clinically verified in multiple university hospitals and has been approved with a sensitivity of 80% or more. Investigators at these hospitals – Freiburg University Hospital, Freiburg, Germany; Massachusetts General Hospital, Boston, Massachusetts; Samsung Medical Center, Seoul, South Korea; and Severance Hospital, Seoul, South Korea – retrospectively identified 600 chest radiographs with lung cancer and 200 normal chest radiographs.
- Extensive external clinical validation that has been recognized by the radiological society for having been performed with ‘unprecedented’ diversity of images acquired under different conditions and demographics, while only producing a small number of false positives per image (0.15).
- Providing an option (Autorun) to automatically perform nodule detection immediately after chest X-ray imaging, and providing PACS transmission options to suit the hospital environment, both which simplify a user’s workflow.
Clinical evaluation results have demonstrated that all readers' nodule detection performances using ALND have increased with statistical significance.
As part of our commitment to advancing diagnostic radiology using AI, Samsung is collaborating with Vuno, a leading developer of AI solutions in healthcare. As part of our collaboration, we will expand the uses of the chest CADe solution and improve the diagnostic accuracy and workflow.
For more information on Samsung’s healthcare business and products, please visit www.SamsungHealthcare.com.
* ALND cannot be used on patients who have lung lesions other than abnormal nodules.
About NeuroLogica
NeuroLogica Corp., the healthcare subsidiary of Samsung Electronics Co., Ltd., develops, manufactures, and markets innovative imaging technologies and is committed to delivering fast, easy and accurate diagnostic solutions to healthcare providers. NeuroLogica, the global corporate headquarters and manufacturer of mobile computed tomography, is also the US headquarters for sales, marketing and distribution of all Samsung digital radiography and ultrasound systems. NeuroLogica’s growing portfolio of advanced medical technologies is used worldwide in leading healthcare institutions helping providers enhance patient care, improve patient satisfaction, and increase workflow efficiency. For more information, please visit http://www.NeuroLogica.com.
Media Inquiries
Lynne Gagne
NeuroLogica
978.564.8576
lgagne@neurologica.com






